About Event
With the recent release of USP General Chapter <1037> Process Analytical Technology – Theory and Practice and the anticipated FDA guidelines on PAT expected in July, 2025 marks a pivotal year for biotech and pharma companies to stay current with the latest advancements in PAT and integrate them into their operations.
Connecting 80+ PAT experts in pharma and biopharma, this year's summit will address the challenges of high initial investment costs, integration with existing systems, and data management, while exploring the rapid growth of PAT applications in biopharmaceuticals, personalized medicine, and biologics.
Join the opportunity to connect with managers and directors of PAT from large pharma, top biotechs and leading CDMOs a the forefront of process innovation as they discuss cutting-edge innovations, practical solutions, and the strategic roadmaps to successfully navigate the future of PAT in pharma and biopharma
Join Your Peers to:
Benchmark your strategies against industry leaders from top pharma companies, biotechs, and CDMOs, and explore real-world solutions and cutting-edge technologies that are shaping the future of PAT.
Gain actionable insights to overcome tech transfer challenges for small molecule and complex therapy manufacturing processes by hearing real-world case studies on how drug manufacturers are successfully implementing PAT.
Understand New FDA Guidelines on PAT for Drug Development & Manufacturing by hearing from regulatory and industry experts on how to align your strategies for smoother approvals and compliance with GMP standards.
Demonstrate the value of PAT to key stakeholders and gain executive buy-in by learning from top pharma and biotech companies that are demonstrating ROI through reduced batch failures, faster release timelines, and enhanced process efficiency.
Discover how leading pharma and biotech teams are leveraging PAT to move towards Real-Time Release Testing, driving faster production timelines while maintaining high-quality standards.
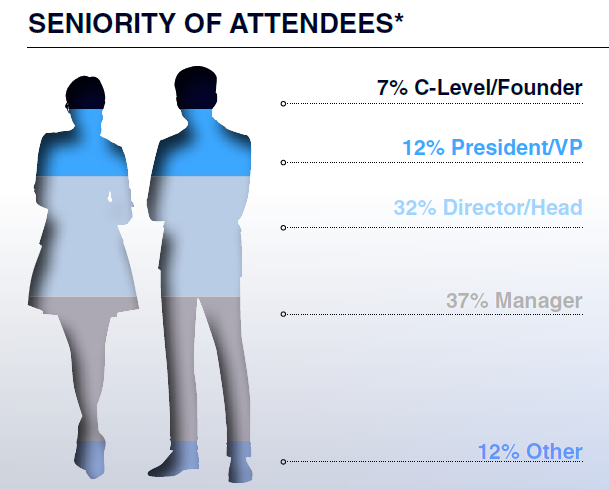
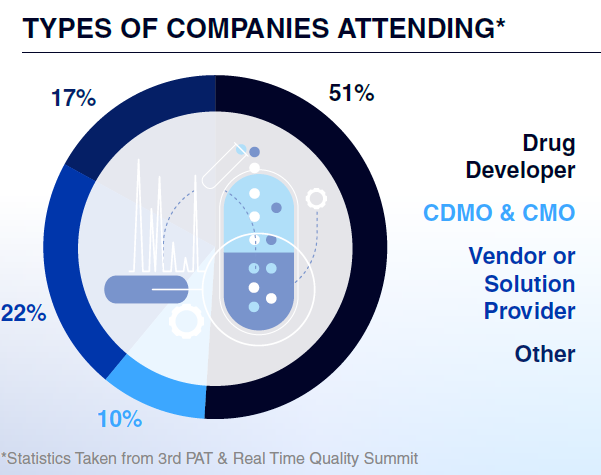
Who Will You Meet?
What Your Peers Have to Say:
"Was great to have the chance to be with leading PAT practitioners and exchange and discuss about the current trends, opportunities and challenges in the pharma business. I enjoyed many high-quality talks."
Head of Advanced Analytical Technologies, Roche
"The great opportunity to meet with PAT experts and share the advances and usage of PAT and chemometrics models within my company. Delighted to have learned more on other platforms such as Biotech and Cell and Gene Therapies."
Global Statistical & PAT Lead, Novartis
"Well attended with representatives from companies that are highly influential in the PAT space. Small enough of a show to make networking with others comfortable. Plenty of opportunities to share thoughts with others."
Sales & Business Development Manager, Optimal Industrial Technologies
"Unlike many other professional conferences I have attended, this one had a much more collaborative environment. The information was helpful, but the peer insight was unique at this event."
Senior Principal Development Engineer, Noven Pharmaceuticals


